The name carbohydrate indicate chemical composition of sugars as carb means carbon and hydrate means hydrogen and oxygen. Carbohydrates have been classified in recent years on the basis of carbohydrate structures not their formulae.

Carbohydrates Definition Classification Sources Importance Geeksforgeeks
Carbohydrate indicates the presence of carbon hydrogen and oxygen of which sugar molecule is made up of.

. They are called carbohydrates as they comprise carbon hydrogen and oxygen at their chemical level. Carbohydrates also called carbs are a type of macronutrient found in certain foods and drinks. The chemical formula of a carbohydrate is C x H 2 O y which denotes some carbons C with some water molecules H 2 O attachedhence the.
Why does this combination correctly describes this chemical group. However not all carbohydrates conform to this precise stoichiometric definition nor are all chemicals that do conform to this definition automatically classified as carbohydrates. This classification is as follow.
Under aerobic conditions pyruvate enters the Krebs cycle also called the citric acid cycle or tricarboxylic acid cycle. Glucose has a carbonyl carbon at carbon number one and can be formed into a five- or six-membered ring. Carbohydrate chains come in different lengths and biologically important carbohydrates.
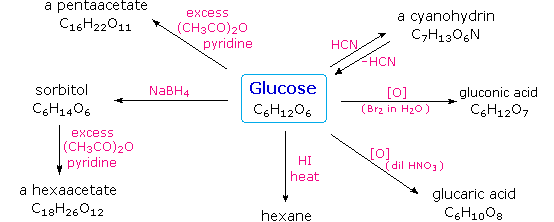
Carbohydrates are biological molecules made of carbon hydrogen and oxygen in a ratio of roughly one carbon atom to one water molecule. Its chemical formula is C₆H₁₂O₆. The word carbohydrate refers to a compound of carbon hydrogen and oxygen that is a major source of energy for animals.
A carbohydrate is an aldehyde or a ketone that has additional hydroxyl groups. Why does the catabolism of fat produce more energy than an equivalent mass of carbohydrate. Therefore the ratio of carbon to hydrogen to oxygen is 121 in carbohydrate molecules.
They are made up of carbon carbo - plus water - hydrate. Carbohydrates can be represented by the stoichiometric formula CH 2 O n where n is the number of carbons in the molecule. The word carbohydrate is derived from carbon and water hydrate.
In chemistry carbohydrates are a common class of simple organic compounds. Originally the term carbohydrate was used to describe compounds that were literally carbohydrates because they had the empirical formula CH 2 O. Because the general formula of cabohydrates is Ca H2Ob.
Why does this correctly describe this chemical group. The origin of the term carbohydrate is based on its components. Carbon carbo and water hydrate.
Why does this combination correctly describes this chemical group. B Carbohydrate catabolism involves substrate-level phosphorylation. Complex polysaccharides containing an amino group.
The name and its origin is appropriate because the formula for a carbohydrate is Cn H₂On. The words carbohydrate is derived from carbon and water hydrate. Some common examples are glucose Ribose etc.
Carbohydrates containing an aldehyde group are called aldoses C12 and 3 and those called ketoses c1 2 and 3. The chemical formula of a carbohydrate is CxH2Oy which denotes some carbons C with some water molecules H2O attachedhence the word carbohydrate which means hydrated carbon. These are the simplest form of carbohydrate that cannot be hydrolyzed any further.
The chemical structure of carbohydrates is present in both linear and ring form. A more correct name used generally in Europe is glucides. Carbohydrates that on hydrolysis yield two to ten smaller units or monosaccharides are oligosaccharides.
This composition gives carbohydrates their name. A carbohydrate is an organic molecules containing long chains of C H and O molecules. Any monosaccharide sugar that contains a ketone group or its hemiacetal.
The word carbohydrate comes from the Greek word sakharon which means sugar. Carbo- would indicate the presence of the element carbon while. The word carbohydrate is derived from carbon and water hydrate.
For every carbon there is a water molecule as well thus the hydrate in carbohydrate. Why does the combination of carbon and water correctly describe carbohydrates. Galactose is a monosaccharide and has the same chemical formula as glucose ie C 6 H 12 O 6.
Occur chiefly as components of connective tissue. As shown below an aldose has the carbonyl CO group at. The name carbohydrate gives away the primary components of its chemical makeup.
Carbohydrates are macronutrients and are one of the three main ways by which our body obtains its energy. The term is most. This is the basic group of a carbohydrate.
Carbohydrates are more descriptively defined as aldehyde or ketone compounds with multiple hydroxyl groups. What elements are present in the amino acids that were not present in carbohydrates. The first step of carbohydrate catabolism is glycolysis which produces pyruvate NADH and ATP.
Under anaerobic conditions the pyruvate can be converted into lactate to keep glycolysis working. A carbohydrate is a biomolecule consisting of carbon hydrogen and oxygen atoms usually with a hydrogenoxygen atom ratio of 21 and thus with the empirical formula CmH2On. Carbohydrate can be defined as naturally occuring molecules that is made of carbon hydrogen and oxygen.
They have the general formula of CH 2 O n. Examples of carbohydrates are sugars like glucose. Such aldehydes and ketones are now known as polyhydroxy.
Carbohydrates are essential nutrients which include sugars fibers and starches.

Create A Concept Map Of Biomolecules Concept Map Biology Activity Graphic Organizers

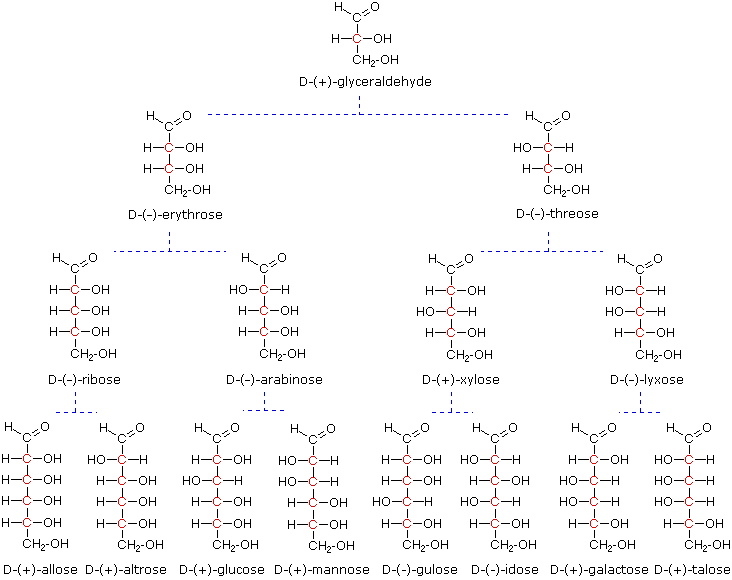
0 Comments